 Abraham Lincoln
If given the truth, the people can be depended upon to meet any national crisis...
Abraham Lincoln
If given the truth, the people can be depended upon to meet any national crisis...
 Guildford news...
for Guildford people, brought to you by Guildford reporters - Guildford's own news service
Guildford news...
for Guildford people, brought to you by Guildford reporters - Guildford's own news service
Letter: Can Anyone Help Develop This Potential Treatment of an Eye Condition that Affects Millions?
Published on: 3 Aug, 2023
Updated on: 6 Aug, 2023
From: Nevin Stewart FRSC
This is a plea for help.
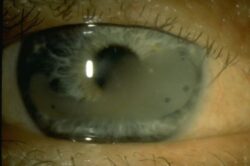
I have spent ten years trying to find a lead medical researcher to progress my proposal for a modern improved treatment of band keratopathy, sadly without success. There have been interested professionals but nothing ever came of these false dawns.
Band keratopathy is a chronic eye condition that, according to a 2022 study published in India, affects 0.33 per cent of the population. That’s over 4.5 million of India’s 1.4 billion people, or nearly 26 million of the world’s 7.8 billion. It occurs as a result of eye trauma and a range of other metabolic dysfunctions.
I believe a simple, non-invasive and continuous treatment based on the daily instillation of eye drops containing an active therapeutic drug could be used to treat the condition. Calcium phosphate scale would be gradually dissolved away and its further ongoing deposition inhibited.
The active agent will be identified by studying biodegradable aspartate and inulin chemistries and other candidate actives such as phosphocitrate, a powerful calcification inhibitor. The former compound classes are known to be effective inorganic scale inhibitors and removers in oilfield production settings.
The current treatments are invasive, can be damaging, and do not prevent recurrence. A successful new eye drop-based treatment will restore vision in affected individuals, maintain optical clarity, avoid potentially damaging and expensive surgery, and prevent disabling partial sight or blindness.
Ten years on from my original idea and I have run out of new contact ideas. Can anyone help please?
Recent Articles
- Guildford Institute’s Crowdfunding Project for Accessible Toilet in its New Community and Wellbeing Centre
- Letter: Guildford – Another Opportunity Missed?
- Letter: GBC’s Corporate Strategy – Where Is the Ambition?
- My Memories of John Mayall at a Ground-breaking Gig in Guildford Nearly Six Decades Ago
- Westborough HMO Plans ‘Losing the Heart of the Street’ Says Resident
- College Invests to Boost Surrey’s Economy and Close Digital Skills Gap
- Community Lottery Brings Big Wins for Local Charities
- GBC Housing Plan Promises ‘A Vibrant Urban Neighbourhood’ Near Town Centre
- Hospital Pillows ‘Shortage’ at the Royal Surrey
- Updated: Caravans Set Up Camp at Ash Manor School


Search in Site
Media Gallery
Dragon Interview: Local Artist Leaves Her Mark At One of England’s Most Historic Buildings
January 21, 2023 / No Comment / Read MoreDragon Interview: Lib Dem Planning Chair: ‘Current Policy Doesn’t Work for Local People’
January 19, 2023 / No Comment / Read MoreA3 Tunnel in Guildford ‘Necessary’ for New Homes, Says Guildford’s MP
January 10, 2023 / No Comment / Read More‘Madness’ for London Road Scheme to Go Ahead Against ‘Huge Opposition’, Says SCC Leader
January 6, 2023 / No Comment / Read MoreCouncillor’s Son Starts Campaign for More Consultation on North Street Plan
December 30, 2022 / No Comment / Read MoreCounty Council Climbs Down Over London Road Works – Further ‘Engagement’ Period Announced
December 14, 2022 / No Comment / Read MoreDragon Interview: GBC Reaction to the Government’s Expected Decision to Relax Housing Targets
December 7, 2022 / No Comment / Read MoreHow Can Our Town Centre Businesses Recover? Watch the Shop Front Debate
May 18, 2020 / No Comment / Read More









Recent Comments